So if you get thirsty at the beach drinking seawater makes you even more dehydrated. The effects of isotonic, hypotonic, and hypertonic extracellular environments on plant and animal cells is the same. moves equally in and out of the cell.
A hypertonic solution contains a high concentration of the solute compared to the solvent molecules. Many animal cells, which lack a cell wall to provide support against the effects of water pressure, rely on the stability of the external environment to maintain their shape. In human cheek cells, the nucleus is located at the _____. 1.65% (m/v) glucose solution c. an animal cell placed in a hypertonic solution will shrink in a process called crenation. A hypertonic solution has increased solute, and a net movement of water outside causing the cell to shrink. Cell wall c. Mitochondrial d. Lysosome 21. The prefix, iso, refers to things that are the same. Center of the cell b. Therefore, plant cells withstand changes in environmental concentration much better than animal cells which burst in such conditions. Isotonic solution: A solution with same osmotic concentration as part of other cell. * No change occurs to an animal cell when kept in an isotonic In addition to the six elements most prevalent in living things there are other elements that are required for human life.
What is Hypertonic. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher . This cell organelle helps organisms maintain homeostasis by controlling what substances may enter or leave cells. A human red blood cell in hypotonic solution When a cell is kept in hypertonic solution it becomes? If the solution is isotonic relative to the cell, then the solute concentrations are the same on both sides of the membrane and water moves equally in both directions. a. Hypotonic Solution vs. Hypertonic Solution. Source: qph.fs.quoracdn.net.  Animal Cell. A hypertonic solution has a greater concentration of non-permeating solutes than another solution. Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes (salts in solution which in this case is represented by body fluid) to keep the body fluids from becoming too diluted or Observe the cells under normal conditions, and make a sketch of what you see. Animal cells 9. What happens when the animal cell is placed in hypotonic solution? If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. Cells are made up of water and solutes suspended or dissolved in it. And covered by a selectively permeable cell membrane which freely allows movem An animal cell placed in a hypotonic solution will swell and potentially burst in a process called hemolysis. An enzyme is a form of DNA. What will happen if an animal cell is placed in hypertonic solution ? Hypotonic-in two solation which have lawer solute con centration is called hypotonic.
Animal Cell. A hypertonic solution has a greater concentration of non-permeating solutes than another solution. Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes (salts in solution which in this case is represented by body fluid) to keep the body fluids from becoming too diluted or Observe the cells under normal conditions, and make a sketch of what you see. Animal cells 9. What happens when the animal cell is placed in hypotonic solution? If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. Cells are made up of water and solutes suspended or dissolved in it. And covered by a selectively permeable cell membrane which freely allows movem An animal cell placed in a hypotonic solution will swell and potentially burst in a process called hemolysis. An enzyme is a form of DNA. What will happen if an animal cell is placed in hypertonic solution ? Hypotonic-in two solation which have lawer solute con centration is called hypotonic.
So if you get thirsty at the beach drinking seawater makes you even more dehydrated. b) Hypertonic solution. Since the cell sap has a lower water potential than that of the solution outside the living cell, Isotonic solution. If an animal cell is placed in a hypotonic solution then the cell will get swell as the solvent content is more in hypotonic solution so the water will come inside the cell. April 4, 2017. Weegy: Isotonic environment results from an equal amount of solute and solvent in and out of the cell.User: 14. Through this process, animal cells would absorb more water The solution outside the cell is what we are referring to when we talk about isotonic, hypertonic, or hypotonic. False. The left side of the cell c. It has the same concentration of solute, and so you have no net inflow. Procedure: Prepare a wet mount of one leaf from the water plant Elodea using the water in which it is kept. an animal cell placed in a hypertonic solution will _____. Weegy: Isotonic environment results from an equal amount of solute and solvent in and out of the cell.User: 14. moves from the solution to the inside of the cell. moves from the inside of the cell to the solution. Source: i.ytimg.com. Unlike an animal cell, the plant cell does not burst. However, some cases call for a hypotonic solution. A sample of cells is placed in a salt solution. Osmosis through a animal cell: Solution tonicity may be manipulated to exert extreme osmotic stress on component cells of source tissues. Horticulture.
A) cell A B) cell BC) cell C D) cell D E) cell E. 15. If the medium is hypertonic relative to the cell cytoplasm, the cell will lose water by osmosis. The effects of osmosis on animal and plant cells Animal cells. Estimation of osmolarity in tissues by bathing samples in hypotonic and. Answer (1 of 13): A solution which has a lower osmotic concentration (high water potential) than another solution is said to be hypotonic. Kidneys that contain more minerals and waste than liquid are said to be _____ to normal kidneys, because they _____. In animal cells, being in a hypertonic environment results in crenation, where the shape of the cell becomes distorted and wrinkled as water when we keep an animal cell in hypertonic solution it shrinks because it have relatively low osmotic pressure as compared to the outside solution which. In addition to the six elements most prevalent in living things there are other elements that are required for human life. Plant cells placed in a hypertonic solution will undergo plasmolysis, a animal cells placed in a hypertonic solution will undergo crenation, a condition where the cell shrivels up as it loses water. hypertonic: An animal cell placed in which type of solution will swell (and possibly burst) as water enters the cell? The Elodea cells have been placed in a 10% NaCl solution. Crenation can be seen in animal cell (primarily RBC). True/False 21) Discuss the following: exocytosis, endocytosis, phagocytosis, pinocytosis, and.
Animal cells do not have rigid cell walls like plants and the cell wall is unable to prevent permeation by the hypotonic solution. In the study animal, red blood cells were used as the experimental cells. Placing an animal cell in a hypertonic solution will cause water to move into the cell. In an isotonic environment, there is no net water movement, so there is no change in the size of the cell. protein. 2.5% (hypotonic), 5% (isotonic), 20% and 50% (hypertonic). Source: d2vlcm61l7u1fs.cloudfront.net. Hypertonic solutions cause cells to shrivel and shrink in size, which can cause problems and inhibit proper cell functioning.
Marinescu et al. A cell placed in a solution with higher salt concentration, on the other hand, tends to make the membrane shrivel up due to loss of water into the hypertonic or high salt environment. The cell wall can withstand dilute hypotonic media and thus it prevents bursting of cells. Check the cells that are eukaryotic Bacteria ArchaeaAnimal Plant Algae ProtistFungi (molds, yeasts and mushrooms) Helminths Viruses.
The cells swell then burst. lose water by osmosis and shrivel gain water by osmosis and lyse gain water by pinocytosis and lyse lose water by active transport and shrivel Plant cells placed in a hypertonic solution will undergo plasmolysis, a condition where the plasma membrane pulls away from the cell wall as the cell shrinks. The solution may be pure water or the solution may be water with a solute dissolved in it, or any such solution. The cells swell then burst. If a cell is placed in a hypertonic solution, the cell is considered Quiz. Although some effects can be seen, the rigid cell wall can hide the magnitude of what is going on inside.. Osmosis and Diffusion. This occurs because of osmosis. Osmosis has different meanings in biology and An animal cell placed in a hypertonic solution will shrink in a process called crenation. a hypotonic solution on cells using terms from class related to tonicity and osmolarity (example: effect of seawater vs . In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of a cell membrane; a solution outside of a cell is called hypertonic if it has a greater concentration of solutes than the cytosol inside the cell. A cell placed in this solution will give up water (osmosis) and shrink. Q. Tags: Question 8 . When this happens, the osmotic gradient causes water to rush out of the cell and it becomes wrinkled or shriveled. A cell loses its * H_2O molecules when it is placed in hypertonic solution*. An animal cell placed in a hypotonic solution will swell and potentially burst in a process called hemolysis. Exosmosis is a process in which the water molecules move from inside of the cell of lower solute concentration to the outside of the cell of higher solute concentration through the cell membrane. 2.5% (hypotonic), 5% (isotonic), 20% and 50% (hypertonic). Osmosis is the diffusion of water across a selectively permeable membrane. A hypertonic solution contains a higher concentration of solutes compared to another solution. 14. hypotonic Sketch your observations. (2 pt) The diffusion of water across a selectively permeable membrane is called A. diffraction B.osmosis C. carbonation D. permeation. When molecules move down the concentration gradient it means they are moving from _ a. Dehydration and hypernatremia can be treated with hypotonic solutions, while bleeding can be treated with hypertonic > solutions. inside a cell versus outside the cell). Hypertonic solutions make plant cells lose water. As seen in Figure 4.2, a cell placed in water tends to swell due to gain of water from the hypotonic or low salt environment. When a cell is placed in hypertonic solution, it will loose water through osmosis ( as water always moves from an area of low solute concentration to an area where solute concentration is comparatively higher). An animal cell placed in a hypertonic (salty) solution will. a. An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water. Dar et al. A cell loses its * H_2O molecules when it is placed in hypertonic solution*. An animal cell placed in a hypotonic solution will swell and potentially Tonicity is the concentration of a solution as compared to another solution. Cell osmosis, cell in isotonic solution, cell in hypertonic solution, cell in hypotonic solution. Source: d2vlcm61l7u1fs.cloudfront.net. In this case the cell is the hypertonic solution sothere is a smaller percentage of water than the hypotonic solution.When the cell is put in the hypotonic solution the water or fluidmoves into the cell through osmosis or diffusion to If a solution has a higher concentration of solutes (less water) than another it is said to be hypertonic.A hypotonic solution has a lower concentration of solutes and more water than Q. Cell. PHSchool.com was retired due to Adobes decision to stop supporting Flash in 2020. Animal Cell: The cell wall is present: The cell wall is absent: Chloroplast is present: Chloroplast is absent: Vacuoles are large and can occupy 90% of cell space: Ans: When a plant cell is placed in a hypertonic solution, water will move outside the plant cell, i.e. exosmosis takes place. A hypertonic solution is a solution that has a greater concentration of solute compared to the cell and less water. The cell X is _____. Hypotonic solution is a solution which, contains lesser solute concentration. A hypotonic solution is less concentrated than the cell, a hypertonic solution is more concentrated than the cell, and isotonic is balanced between the cell and outside solution. Cells can acquire specified function and carry out various tasks within the cell such as replication, DNA repair, protein synthesis, and 20) Both animal and plant cells are healthiest in hypotonic environments. solution with 5%glucose and 0.9% NaCl is isotonic to all cells. Being isotonic, additon of this solution does not change osmolarity of ICF and ECF. Seawater is hypertonic. To prevent crenation or hemolysis, an animal cell must be placed in an isotonic solution such as 0.9% (m/v) nacl or 5.0% (m/v) glucose. When a cell is placed in a hypertonic (more concentrated) solution water. When a cell is placed in a hypotonic environment, water will enter the cell, and the cell will swell. lipid. So, When cell is placed in this kind of solution then Osmosis through a animal cell: Solution tonicity may be manipulated to exert extreme osmotic stress on component cells of source tissues. What happens to an animal cell in a hypertonic solution? hypertonic The ideal osmotic environment for a plant cell is a(n) _______________ environment. Tonicity is a measure of the relative concentration of solute particles on either side of a semi-permeable membrane (e.g. cell, the water molecules move from inside of the cell to the outer environment by osmosis. Please contact Savvas Learning Company for product support. Some examples for solutions that are isotonic with animal cells are given below. toward the hypertonic solution (the solution with the higher concentration). A cell placed in this solution will take up water (osmosis) and blow up. In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of a cell membrane; a solution outside of a cell is called hypertonic if it has a greater concentration of solutes than the cytosol inside the cell. Beranda Animal Cell In Hypotonic Hypertonic And Isotonic Solution - 21 best images about Isotonic, Hypotonic, Hypertonic on / When an animal cell is in a hypotonic solution, that means that the outside liquid has less solute isotonic solution is a solution in which the concentration of water is the same as that of the cell. Concentration describes the amount of solutes dissolved by a solution. Clean slides and covers were used to hold the cells and while 10% sodium chloride solution was used to make up the hypertonic solution. The animal cell will die and may burst also. Cell-free DNA screening for prenatal detection of 22q11.2 deletion syndrome. A Hypotonic solution has less solute than the cell. Therefore, a hypertonic solution has more solutes than the intracellular environment, so water will leave the cell to try to achieve equilibrium. 19) What will happen to an animal cell when placed in a/an: a) hypertonic solution, b) hypotonic solution, c) isotonic solution. The opposite solution with a lower concentration is known as the hypotonic solution.Scientists must describe cell contents compared to the environment. glucose. Therefore the solution it is placed in must have the same concentration of water as the cell, or osmosis will take place. In order to grasp the mechanisms of osmosis, one must understand the difference between a hypotonic solution and a hypertonic solution. Plant cell b. Hypertonic solution is the one which have higher concentration than the cell. When we keep an animal cell in hypertonic solution it shrinks because The cell wall is fully permeable to all molecules and supports the cell and stops it bursting when it gains water by osmosis. The cell wall is rigid and does not shrink. The plant and animal cell structures are very much similar because of the presence of eukaryotic cells in both. glucose. If plant cells are placed in solutions of increasing solute concentration: A sample of cells is placed in a salt solution. Peer-reviewed articles cover topics in oncology, trauma, gastrointestinal, vascular, and transplantation surgery.The journal also Hypotonic solution is a solution which, contains lesser solute concentration. Hypotonic solution, you have water molecules going into the cell, the cell expanding, kind of like a filling balloon. An animal cell placed in a hypertonic solution will shrink in a process called crenation. In red blood cells this is called crenation and the surface of the cells take on a scalloped appearance. In animal cells, being in a hypertonic environment results in crenation, where the shape of the cell becomes distorted and wrinkled as water leaves 1. Mid-trimester uterine electromyography in patients with a short cervix. hypotonic: In which type of solution is the net movement (gain) of water molecules equal to zero (0)? When there are solutes on two sides of a Most animals maintain the pH and osmolarity of the fluids inside of their bodies to create isotonic solutions to bathe their cells in. none of these. The contents of the cells have been reduced to the spherical structures shown. So, When cell is placed in this kind of solution then If a cell containing 5% salt is placed into a glass of water with 20% salt, the water is Tonicity Definition. If two solutions are of equal concentration they are isotonic. What is hypertonic solution in short answer? As a result, the cell will shrink.
 Weegy: An enzyme is a form of: C) protein.User: 15. A cell X contains a cell wall, large central vacuole and a nucleus at the periphery. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ). If the medium is hypotonic relative to the cell cytoplasm, the cell will gain water through osmosis. 0% (m/v) glucose concentration; it just means that 5. Isotonic solution, no net flow. Solution A liquid mixture in which a solid component is completely dissolved in a liquid component. If a cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume. If an animal cell is place in a This allows the cells to decrease in its internal pressure and results in shrinking of the cell. hypotonic There is a net diffusion of water out of an animal cell when it is placed in a(n) ____________ solution.
Weegy: An enzyme is a form of: C) protein.User: 15. A cell X contains a cell wall, large central vacuole and a nucleus at the periphery. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ). If the medium is hypotonic relative to the cell cytoplasm, the cell will gain water through osmosis. 0% (m/v) glucose concentration; it just means that 5. Isotonic solution, no net flow. Solution A liquid mixture in which a solid component is completely dissolved in a liquid component. If a cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume. If an animal cell is place in a This allows the cells to decrease in its internal pressure and results in shrinking of the cell. hypotonic There is a net diffusion of water out of an animal cell when it is placed in a(n) ____________ solution. 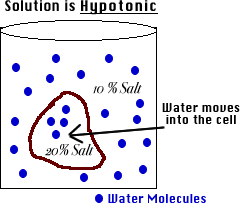
If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher . Hypertonic solution is the one which contain more concentration of solutes as compared to the concentration of solutes in cytoplasm of cell. what is a hiab licence. Hypertonic solution is the one which contain more concentration of solutes as compared to the concentration of solutes in cytoplasm of cell. Solution for How do hypertonic, hypotonic and isotonic solution affect animal cells? It determines the direction and extent to which water moves by osmosis. 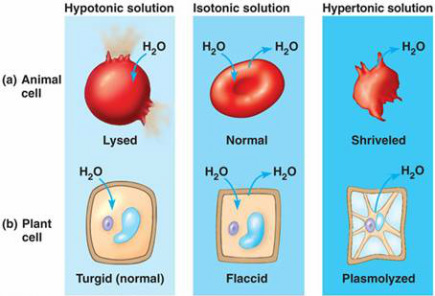 So if you get thirsty at the beach drinking seawater makes you even more dehydrated. 9% (m/v) NaCl or 5. A hypertonic solution contains a high concentration of the solute compared to the solvent molecules. Which basically means, the water molecules are A hypotonic solution means thatthere is more solvent than solute comparitevely to what ever youcompare it to. The Journal of Emergency Medicine is an international, peer-reviewed publication featuring original contributions of interest to both the academic and practicing emergency physician.JEM, published monthly, contains research papers and clinical studies as well as articles focusing on the training of emergency physicians and on the practice of emergency List the functions:nucleus nucleolus ribosomes smooth ER rough ER lysosomes peroxisomes chloroplast mitochondrion cytoskeleton. Osmosis is the movement of water between two mediums until each medium is similarly concentrated. A 0.9% NaCl solution is said to be isotonic: when blood cells reside in such a medium, the intracellular and extracellular fluids are in osmotic eq Animal cell: Despite the fundamental similarities between plants and animal cells, there are some striking differences between them. Science; Anatomy and Physiology; Anatomy and Physiology questions and answers; Explain the effect of a hypertonic solution vs . Tonic Solutions A Hypertonic solution has more solute than the cell. A hypertonic solution is the one that contains higher solute concentration as compared to solvent. The cell will loose as water goes out of the cell to maintainequilibrium. Home thermic-Animals who have a constant body temperature are called home thermo cot warmblooded animal. To understand this definition, we A hypertonic solution has a greater concentration of non-permeating solutes than another solution. 0.45 NaCl and 0.25 NaCl are examples of intravenous. While observing the leaf under the microscope, wick a solution of 6% NaCl (sodium chloride) across the slide. Hypertonic solutions have a higher solute concentration. For the below examples, we will use a cell that has a NaCL concentration of 0.9%. If a plant cell is placed in an isotonic solution it will take up and release water at an 10. water in hypertonic solutions causing them to equal rate, because water leaves the cell Question: 6. An animal cell in hypotonic solution When exposed to a hypotonic solution, animal cells will absorb the hypotonic solution (often water) and then burst. 10 % solution of sodium chloride increased the solute concentration in the surround making it higher than that of the cells. 3.2 A Typical Bacterial Cell 1. What will happen if red blood cells are placed in a hypertonic solution? This does not mean that a cell has a 5. 30. When an cell is kept in an hypertonic solution it swells and it can burst also dye to the high pressure of solvent outside cell and less pressure o When the cell is placed in an isotonic solution Nothin will happen. When a cell is placed in a hypotonic solution due to endosmosis the cell will d When a cell is kept in a hypertonic solution, it shrinks and exosmosis occurs. pure/distilled water on cells in your body)?. Hypotonic solutions induce the cell to swell by allowing water to flow into it, whereas hypertonic solutions cause the cell to shrink by drawing water out of it. A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. Suppose an animal or a plant cell is placed in a solution of sugar or salt in water. answer choices. For 66 years, Surgery has published practical, authoritative information about procedures, clinical advances, and major trends shaping general surgery.Each issue features original scientific contributions and clinical reports. Hypertonic-when two solution have differcut sdute concentration. What is hypertonic solution in short answer? (2pt) Which figure depicts an animal cell placed in a solution hypertonic to the cell? Answer to Solved An animal cell placed in a hypertonic solution will. Distinguish a typical bacterial cell from a typical plant or animal cell in terms of cell shapes and arrangements, size, and cell structures cell wall protects from lysis Hypertonic environments solute concentration outside the cell is greater than inside This process is turgidity, or we call this swelled cell a turgid cell. It increases its fluid intake. 31. Isotonic An animal cell placed in a(n) ______________ solution will gain water, swell, and possibly burst. Through observation of plasmolysis and deplasmolysis, it is possible to determine the tonicity of the cell's environment However, due to the cell walls of plants, the visible effects differ.
So if you get thirsty at the beach drinking seawater makes you even more dehydrated. 9% (m/v) NaCl or 5. A hypertonic solution contains a high concentration of the solute compared to the solvent molecules. Which basically means, the water molecules are A hypotonic solution means thatthere is more solvent than solute comparitevely to what ever youcompare it to. The Journal of Emergency Medicine is an international, peer-reviewed publication featuring original contributions of interest to both the academic and practicing emergency physician.JEM, published monthly, contains research papers and clinical studies as well as articles focusing on the training of emergency physicians and on the practice of emergency List the functions:nucleus nucleolus ribosomes smooth ER rough ER lysosomes peroxisomes chloroplast mitochondrion cytoskeleton. Osmosis is the movement of water between two mediums until each medium is similarly concentrated. A 0.9% NaCl solution is said to be isotonic: when blood cells reside in such a medium, the intracellular and extracellular fluids are in osmotic eq Animal cell: Despite the fundamental similarities between plants and animal cells, there are some striking differences between them. Science; Anatomy and Physiology; Anatomy and Physiology questions and answers; Explain the effect of a hypertonic solution vs . Tonic Solutions A Hypertonic solution has more solute than the cell. A hypertonic solution is the one that contains higher solute concentration as compared to solvent. The cell will loose as water goes out of the cell to maintainequilibrium. Home thermic-Animals who have a constant body temperature are called home thermo cot warmblooded animal. To understand this definition, we A hypertonic solution has a greater concentration of non-permeating solutes than another solution. 0.45 NaCl and 0.25 NaCl are examples of intravenous. While observing the leaf under the microscope, wick a solution of 6% NaCl (sodium chloride) across the slide. Hypertonic solutions have a higher solute concentration. For the below examples, we will use a cell that has a NaCL concentration of 0.9%. If a plant cell is placed in an isotonic solution it will take up and release water at an 10. water in hypertonic solutions causing them to equal rate, because water leaves the cell Question: 6. An animal cell in hypotonic solution When exposed to a hypotonic solution, animal cells will absorb the hypotonic solution (often water) and then burst. 10 % solution of sodium chloride increased the solute concentration in the surround making it higher than that of the cells. 3.2 A Typical Bacterial Cell 1. What will happen if red blood cells are placed in a hypertonic solution? This does not mean that a cell has a 5. 30. When an cell is kept in an hypertonic solution it swells and it can burst also dye to the high pressure of solvent outside cell and less pressure o When the cell is placed in an isotonic solution Nothin will happen. When a cell is placed in a hypotonic solution due to endosmosis the cell will d When a cell is kept in a hypertonic solution, it shrinks and exosmosis occurs. pure/distilled water on cells in your body)?. Hypotonic solutions induce the cell to swell by allowing water to flow into it, whereas hypertonic solutions cause the cell to shrink by drawing water out of it. A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. Suppose an animal or a plant cell is placed in a solution of sugar or salt in water. answer choices. For 66 years, Surgery has published practical, authoritative information about procedures, clinical advances, and major trends shaping general surgery.Each issue features original scientific contributions and clinical reports. Hypertonic-when two solution have differcut sdute concentration. What is hypertonic solution in short answer? (2pt) Which figure depicts an animal cell placed in a solution hypertonic to the cell? Answer to Solved An animal cell placed in a hypertonic solution will. Distinguish a typical bacterial cell from a typical plant or animal cell in terms of cell shapes and arrangements, size, and cell structures cell wall protects from lysis Hypertonic environments solute concentration outside the cell is greater than inside This process is turgidity, or we call this swelled cell a turgid cell. It increases its fluid intake. 31. Isotonic An animal cell placed in a(n) ______________ solution will gain water, swell, and possibly burst. Through observation of plasmolysis and deplasmolysis, it is possible to determine the tonicity of the cell's environment However, due to the cell walls of plants, the visible effects differ.
Weegy: An enzyme is a form of: C) protein.User: 15. American Journal of Obstetrics & Gynecology Vol. Only solutes that cannot cross the membrane contribute to tonicity. Consequently, the water will flow across the membrane and into the dialysis tube. The cell wall can avoid the cell bursting. Original Research Obstetrics. Water inside the cell (highest concentration) moves out of the cell (lowest. When a cell is kept in hypertonic solution it becomes? Animal cell c. Bacterial cell d. Prokaryotic cell 22. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ). Dehydration and hypernatremia can be treated with hypotonic solutions, while bleeding can be treated with hypertonic solutions. Virtual Lab: The Purification of Hemoglobin Participate in a virtual lab to explain what happens at the molecular level when a cell is placed in hypotonic or hypertonic solutions, explain why plant cells thrive in hypotonic solutions while animal cells may rupture, and describe the biological consequences of water gain or loss in a cell The. This is because plant cells have a rigid cell wall around the plasma membrane. During the Cell Homeostasis Virtual Lab, dialysis tube C is placed into a hypotonic solution in beaker C. The fluid in the dialysis tube is hypertonic compared to the fluid in the beaker. To prevent crenation or hemolysis, an animal cell must be placed in an isotonic solution such as 0.9% (m/v) NaCl or 5.0% (m/v) glucose. But incase of plant cell, the cell will die but not burst due to the presence of cell wall. Water inside the cell (highest concentration) moves out of the cell (lowest. Since hypertonic solutions contains higher concentrations for e.g. Hypotonic solutions induce the cell to swell by allowing water to flow into it, whereas hypertonic solutions cause the cell to shrink by drawing water out of it. compare scripture with scripture verse codependency worksheets for group therapy; porsche 914 transmission numbers sterling submersible pumps; cs 1104 vanderbilt courthouse in hazlehurst ms The process in which a cell takes in bulky material, like a macromolecule. What will happen if red blood cells are placed in a hypertonic solution? The effects of osmosis on animal and plant cells Animal cells. Our cells are surrounded by a semi-permeable membrane that allows certain things to move in and out. If enough water is lost, the cell will take on a wrinkled or shriveled appearance. When an animal cell is placed in a hypertonic solution, water diffuses across the selectively permeable cell membrane in an attempt to form an equilibrium between the two liquids. (b) hypertonic solution - Both the animal and the plant cell will undergo plasmolysis.
Due to exosmosis, both animal and plant cells will shrink. The cell (from the Latin word cellula meaning 'small room') is the basic structural and functional unit of life forms.Every cell consists of a cytoplasm enclosed within a membrane, which contains many biomolecules such as proteins and nucleic acids.. When plant cells are placed in such solutions, water will move from inside the plant cell to the outside of the cell, resulting in the shrinking of the cell (the cell is said to be plasmolyzed). To prevent crenation or hemolysis, an animal cell must be placed in an isotonic solution such as 0.9% (m/v) NaCl or 5.0% (m/v) glucose. HYPERTONIC - THE CELL WILL SHIRK BECAUSE THE SOLUTION HAVE LOW CONCENTRATION AND THE MOLECULES FROM THE CELL WILL DIFFUSE(osmosis) TO THE SOLUTION. If animal and plant cells are kept in hypertonic solution then exosmosis will occur. 227 Issue 1 p79.e1. An animal cell placed in a hypertonic solution will _____. Cell osmosis, cell in isotonic solution, cell in hypertonic solution, cell in hypotonic solution. Plasmolysis is the process in which cells lose water in a hypertonic solution. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ). February 12, 2018. Q. Answer (1 of 1): Animal cells prefer immersion in isotonic solutions as they do not have cell walls. Hypertonic solution has more salt concentration and less concentration of water According to osmosis the solvent moves from low to high concentrat In this case, water will leave the cell since the cell has a lower osmolarity than the extracellular fluid. When a cell is put in a hypertonic solution, water escapes the cell and flows into the surrounding solution, causing the cell to shrink and lose its turgidity.
 Animal Cell. A hypertonic solution has a greater concentration of non-permeating solutes than another solution. Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes (salts in solution which in this case is represented by body fluid) to keep the body fluids from becoming too diluted or Observe the cells under normal conditions, and make a sketch of what you see. Animal cells 9. What happens when the animal cell is placed in hypotonic solution? If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. Cells are made up of water and solutes suspended or dissolved in it. And covered by a selectively permeable cell membrane which freely allows movem An animal cell placed in a hypotonic solution will swell and potentially burst in a process called hemolysis. An enzyme is a form of DNA. What will happen if an animal cell is placed in hypertonic solution ? Hypotonic-in two solation which have lawer solute con centration is called hypotonic.
Animal Cell. A hypertonic solution has a greater concentration of non-permeating solutes than another solution. Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes (salts in solution which in this case is represented by body fluid) to keep the body fluids from becoming too diluted or Observe the cells under normal conditions, and make a sketch of what you see. Animal cells 9. What happens when the animal cell is placed in hypotonic solution? If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. Cells are made up of water and solutes suspended or dissolved in it. And covered by a selectively permeable cell membrane which freely allows movem An animal cell placed in a hypotonic solution will swell and potentially burst in a process called hemolysis. An enzyme is a form of DNA. What will happen if an animal cell is placed in hypertonic solution ? Hypotonic-in two solation which have lawer solute con centration is called hypotonic.  Weegy: An enzyme is a form of: C) protein.User: 15. A cell X contains a cell wall, large central vacuole and a nucleus at the periphery. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ). If the medium is hypotonic relative to the cell cytoplasm, the cell will gain water through osmosis. 0% (m/v) glucose concentration; it just means that 5. Isotonic solution, no net flow. Solution A liquid mixture in which a solid component is completely dissolved in a liquid component. If a cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume. If an animal cell is place in a This allows the cells to decrease in its internal pressure and results in shrinking of the cell. hypotonic There is a net diffusion of water out of an animal cell when it is placed in a(n) ____________ solution.
Weegy: An enzyme is a form of: C) protein.User: 15. A cell X contains a cell wall, large central vacuole and a nucleus at the periphery. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ). If the medium is hypotonic relative to the cell cytoplasm, the cell will gain water through osmosis. 0% (m/v) glucose concentration; it just means that 5. Isotonic solution, no net flow. Solution A liquid mixture in which a solid component is completely dissolved in a liquid component. If a cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume. If an animal cell is place in a This allows the cells to decrease in its internal pressure and results in shrinking of the cell. hypotonic There is a net diffusion of water out of an animal cell when it is placed in a(n) ____________ solution. 
 So if you get thirsty at the beach drinking seawater makes you even more dehydrated. 9% (m/v) NaCl or 5. A hypertonic solution contains a high concentration of the solute compared to the solvent molecules. Which basically means, the water molecules are A hypotonic solution means thatthere is more solvent than solute comparitevely to what ever youcompare it to. The Journal of Emergency Medicine is an international, peer-reviewed publication featuring original contributions of interest to both the academic and practicing emergency physician.JEM, published monthly, contains research papers and clinical studies as well as articles focusing on the training of emergency physicians and on the practice of emergency List the functions:nucleus nucleolus ribosomes smooth ER rough ER lysosomes peroxisomes chloroplast mitochondrion cytoskeleton. Osmosis is the movement of water between two mediums until each medium is similarly concentrated. A 0.9% NaCl solution is said to be isotonic: when blood cells reside in such a medium, the intracellular and extracellular fluids are in osmotic eq Animal cell: Despite the fundamental similarities between plants and animal cells, there are some striking differences between them. Science; Anatomy and Physiology; Anatomy and Physiology questions and answers; Explain the effect of a hypertonic solution vs . Tonic Solutions A Hypertonic solution has more solute than the cell. A hypertonic solution is the one that contains higher solute concentration as compared to solvent. The cell will loose as water goes out of the cell to maintainequilibrium. Home thermic-Animals who have a constant body temperature are called home thermo cot warmblooded animal. To understand this definition, we A hypertonic solution has a greater concentration of non-permeating solutes than another solution. 0.45 NaCl and 0.25 NaCl are examples of intravenous. While observing the leaf under the microscope, wick a solution of 6% NaCl (sodium chloride) across the slide. Hypertonic solutions have a higher solute concentration. For the below examples, we will use a cell that has a NaCL concentration of 0.9%. If a plant cell is placed in an isotonic solution it will take up and release water at an 10. water in hypertonic solutions causing them to equal rate, because water leaves the cell Question: 6. An animal cell in hypotonic solution When exposed to a hypotonic solution, animal cells will absorb the hypotonic solution (often water) and then burst. 10 % solution of sodium chloride increased the solute concentration in the surround making it higher than that of the cells. 3.2 A Typical Bacterial Cell 1. What will happen if red blood cells are placed in a hypertonic solution? This does not mean that a cell has a 5. 30. When an cell is kept in an hypertonic solution it swells and it can burst also dye to the high pressure of solvent outside cell and less pressure o When the cell is placed in an isotonic solution Nothin will happen. When a cell is placed in a hypotonic solution due to endosmosis the cell will d When a cell is kept in a hypertonic solution, it shrinks and exosmosis occurs. pure/distilled water on cells in your body)?. Hypotonic solutions induce the cell to swell by allowing water to flow into it, whereas hypertonic solutions cause the cell to shrink by drawing water out of it. A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. Suppose an animal or a plant cell is placed in a solution of sugar or salt in water. answer choices. For 66 years, Surgery has published practical, authoritative information about procedures, clinical advances, and major trends shaping general surgery.Each issue features original scientific contributions and clinical reports. Hypertonic-when two solution have differcut sdute concentration. What is hypertonic solution in short answer? (2pt) Which figure depicts an animal cell placed in a solution hypertonic to the cell? Answer to Solved An animal cell placed in a hypertonic solution will. Distinguish a typical bacterial cell from a typical plant or animal cell in terms of cell shapes and arrangements, size, and cell structures cell wall protects from lysis Hypertonic environments solute concentration outside the cell is greater than inside This process is turgidity, or we call this swelled cell a turgid cell. It increases its fluid intake. 31. Isotonic An animal cell placed in a(n) ______________ solution will gain water, swell, and possibly burst. Through observation of plasmolysis and deplasmolysis, it is possible to determine the tonicity of the cell's environment However, due to the cell walls of plants, the visible effects differ.
So if you get thirsty at the beach drinking seawater makes you even more dehydrated. 9% (m/v) NaCl or 5. A hypertonic solution contains a high concentration of the solute compared to the solvent molecules. Which basically means, the water molecules are A hypotonic solution means thatthere is more solvent than solute comparitevely to what ever youcompare it to. The Journal of Emergency Medicine is an international, peer-reviewed publication featuring original contributions of interest to both the academic and practicing emergency physician.JEM, published monthly, contains research papers and clinical studies as well as articles focusing on the training of emergency physicians and on the practice of emergency List the functions:nucleus nucleolus ribosomes smooth ER rough ER lysosomes peroxisomes chloroplast mitochondrion cytoskeleton. Osmosis is the movement of water between two mediums until each medium is similarly concentrated. A 0.9% NaCl solution is said to be isotonic: when blood cells reside in such a medium, the intracellular and extracellular fluids are in osmotic eq Animal cell: Despite the fundamental similarities between plants and animal cells, there are some striking differences between them. Science; Anatomy and Physiology; Anatomy and Physiology questions and answers; Explain the effect of a hypertonic solution vs . Tonic Solutions A Hypertonic solution has more solute than the cell. A hypertonic solution is the one that contains higher solute concentration as compared to solvent. The cell will loose as water goes out of the cell to maintainequilibrium. Home thermic-Animals who have a constant body temperature are called home thermo cot warmblooded animal. To understand this definition, we A hypertonic solution has a greater concentration of non-permeating solutes than another solution. 0.45 NaCl and 0.25 NaCl are examples of intravenous. While observing the leaf under the microscope, wick a solution of 6% NaCl (sodium chloride) across the slide. Hypertonic solutions have a higher solute concentration. For the below examples, we will use a cell that has a NaCL concentration of 0.9%. If a plant cell is placed in an isotonic solution it will take up and release water at an 10. water in hypertonic solutions causing them to equal rate, because water leaves the cell Question: 6. An animal cell in hypotonic solution When exposed to a hypotonic solution, animal cells will absorb the hypotonic solution (often water) and then burst. 10 % solution of sodium chloride increased the solute concentration in the surround making it higher than that of the cells. 3.2 A Typical Bacterial Cell 1. What will happen if red blood cells are placed in a hypertonic solution? This does not mean that a cell has a 5. 30. When an cell is kept in an hypertonic solution it swells and it can burst also dye to the high pressure of solvent outside cell and less pressure o When the cell is placed in an isotonic solution Nothin will happen. When a cell is placed in a hypotonic solution due to endosmosis the cell will d When a cell is kept in a hypertonic solution, it shrinks and exosmosis occurs. pure/distilled water on cells in your body)?. Hypotonic solutions induce the cell to swell by allowing water to flow into it, whereas hypertonic solutions cause the cell to shrink by drawing water out of it. A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. Suppose an animal or a plant cell is placed in a solution of sugar or salt in water. answer choices. For 66 years, Surgery has published practical, authoritative information about procedures, clinical advances, and major trends shaping general surgery.Each issue features original scientific contributions and clinical reports. Hypertonic-when two solution have differcut sdute concentration. What is hypertonic solution in short answer? (2pt) Which figure depicts an animal cell placed in a solution hypertonic to the cell? Answer to Solved An animal cell placed in a hypertonic solution will. Distinguish a typical bacterial cell from a typical plant or animal cell in terms of cell shapes and arrangements, size, and cell structures cell wall protects from lysis Hypertonic environments solute concentration outside the cell is greater than inside This process is turgidity, or we call this swelled cell a turgid cell. It increases its fluid intake. 31. Isotonic An animal cell placed in a(n) ______________ solution will gain water, swell, and possibly burst. Through observation of plasmolysis and deplasmolysis, it is possible to determine the tonicity of the cell's environment However, due to the cell walls of plants, the visible effects differ.